What causes DMD?
Dystrophin is an important component of the muscle cell membrane, present in all muscles, including skeletal, cardiac and respiratory muscle.1,2,4,5
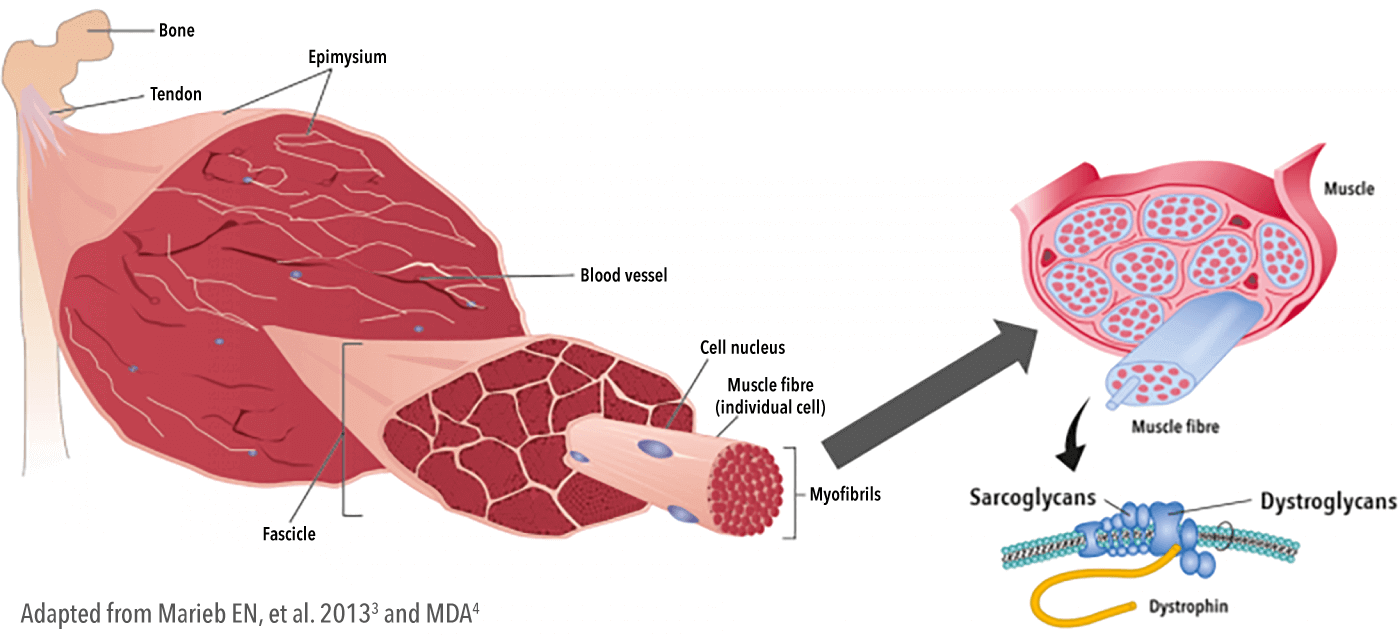
Dystrophin is a structural protein that links the internal cytoskeleton to sarcoglycans/dystroglycans in the membrane and the extracellular matrix, providing mechanical stability and structure to the muscle cell membrane during contraction.5—7
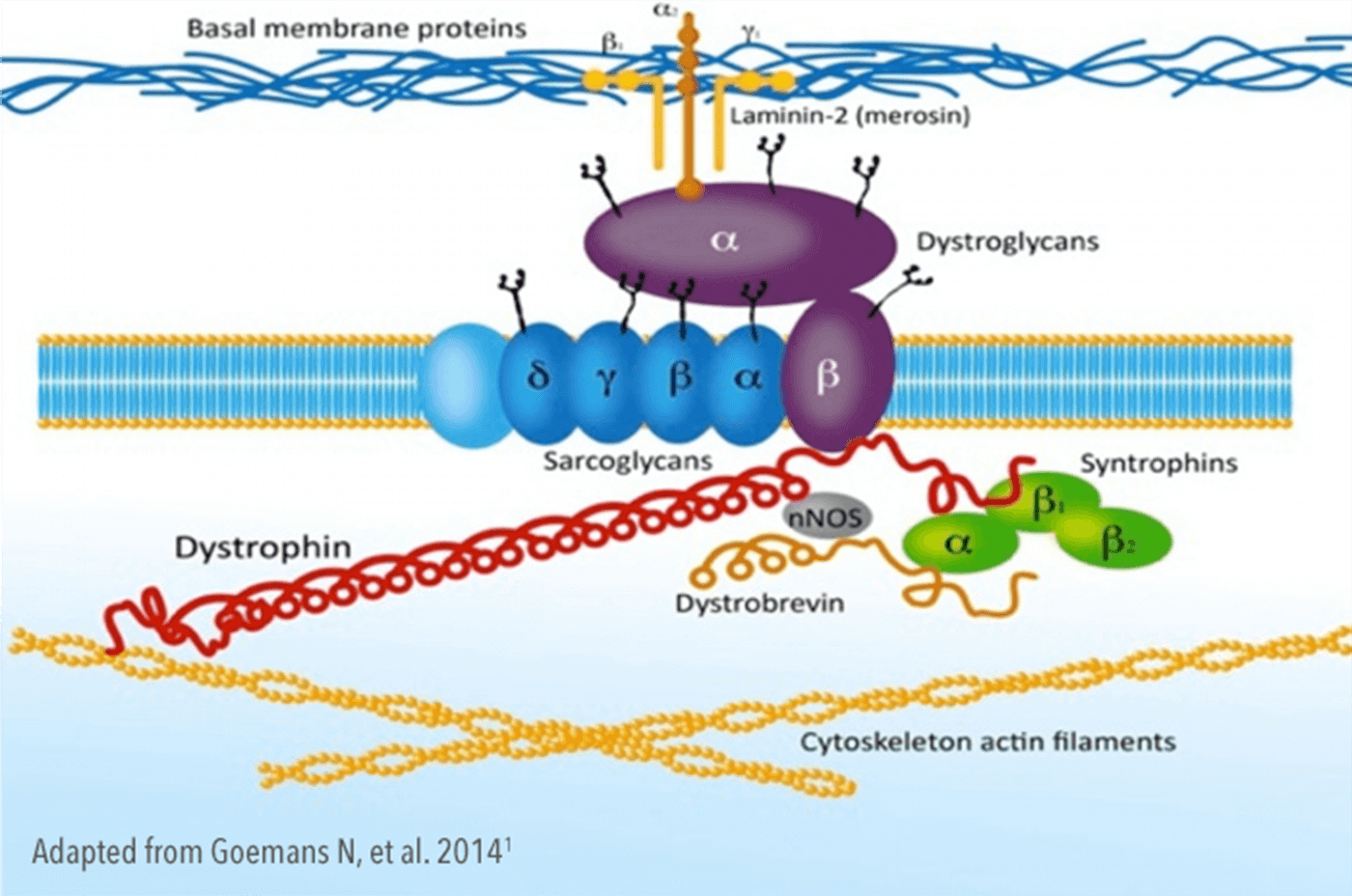
Consequently, the absence of, or defects in, dystrophin leads to muscle degeneration and fibrosis.11,12 Once muscle is lost, it cannot be restored.2,10,12
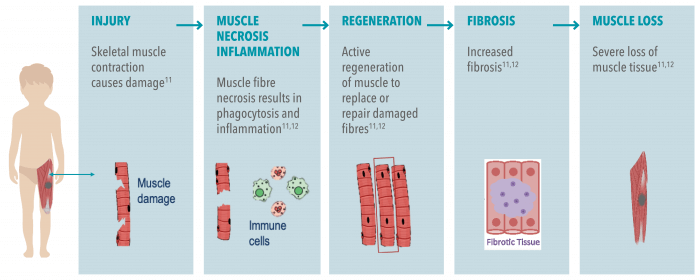
DMD is caused by mutations in the gene encoding dystrophin, resulting in an absence of the protein.12 The lack of dystrophin leads to ongoing muscle damage, and replacement of muscle fibres by scar tissue and fat.12
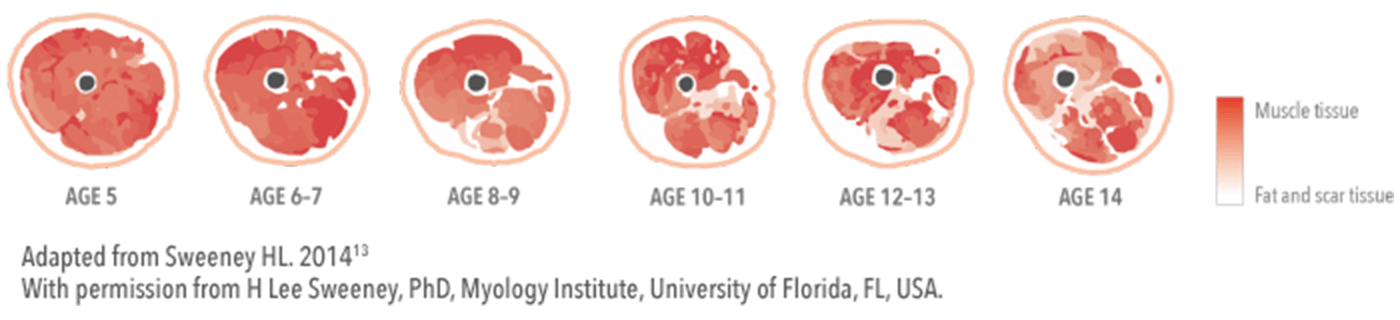
- By the age of 5 years, prominent muscle weakness becomes evident with a 50–60% drop in strength14
- By age 6 years, only 60% of predicted muscle mass is retained, decreasing to just 20% at age 16 years15
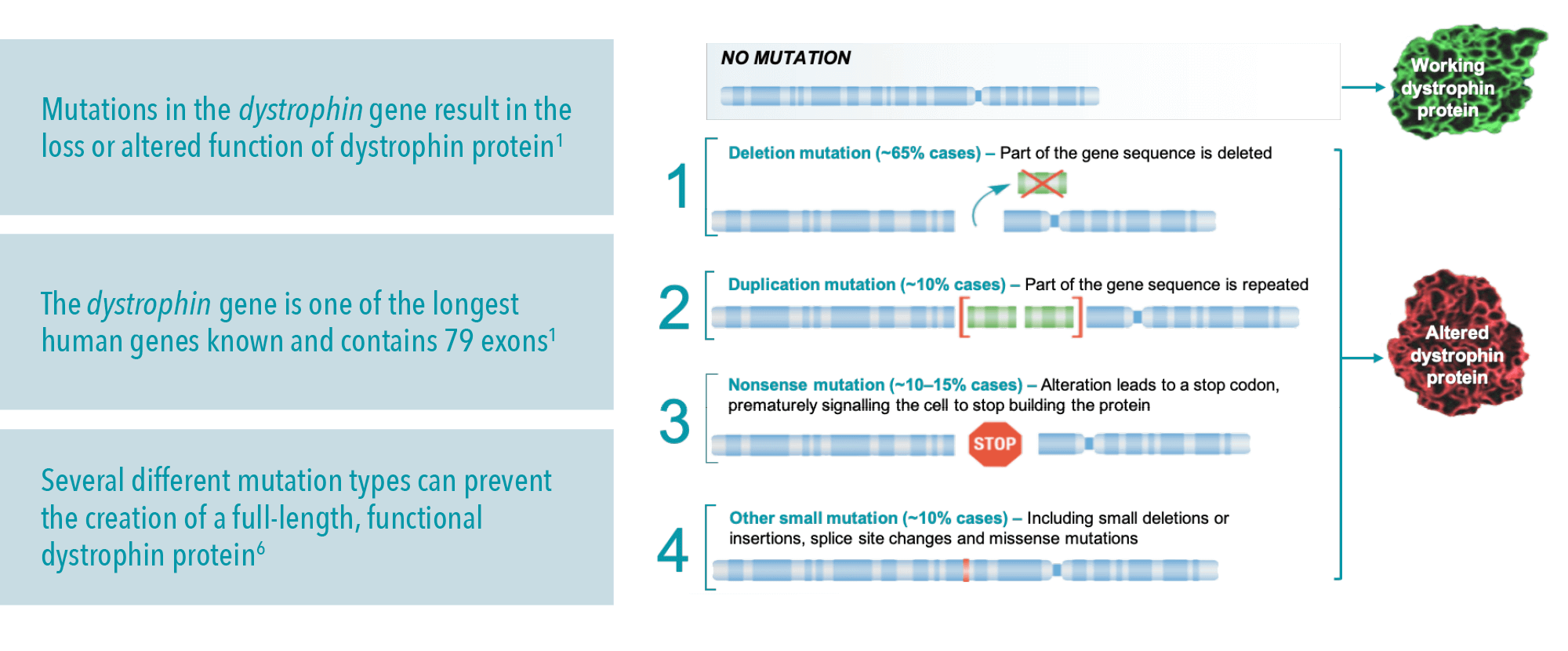
DMD causes: The dystrophin gene
The dystrophin gene is located on the X chromosome and is the largest gene in the human genome, which may make it more susceptible to mutations.16,17 So far, more than 7,000 individual mutations in the dystrophin gene have been identified.16
DMD is caused by deletion, duplication, point and other small mutations in the dystrophin gene.6,16,18
- Knowing the mutation type can be helpful for medical management options, and the possibility of enrolling into clinical trials2,18
- Large mutations can be detected using multiplex ligation-dependent probe amplification. Small deletions, such as nonsense mutations, require gene sequencing18
Only a DMD genetic test can identify the dystrophin gene mutation type; this is important for genetic counselling, prenatal diagnosis and considering mutation-specific therapies.
Learn more about Genetic testing for Duchenne muscular dystrophy.
DMD inheritance
How is DMD inherited?
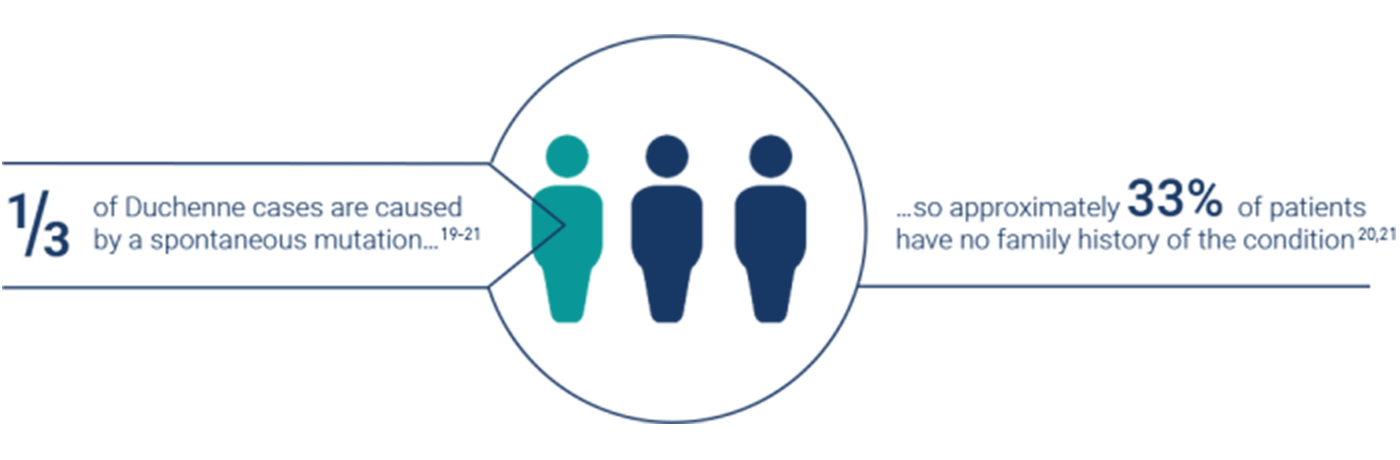
Approximately one-third of DMD cases are thought to arise because of de novo mutations, with the remaining two-thirds of cases inheriting the mutation from carrier mothers.19—21
Why does DMD only affect males?
DMD is inherited in an X-linked recessive pattern, meaning the gene that carries the DMD-causing mutation is on the X chromosome.4 Since males have only one X chromosome, a mutation in the gene responsible for DMD is sufficient to cause the condition.4
DMD mode of inheritance
In X-linked recessive inheritance, a female with one mutated copy of the gene can pass it on to her children. Every son and daughter of a female carrier has a 50% chance of inheriting the faulty gene. Sons who inherit the faulty gene will have DMD, while daughters will be carriers.4
Carriers of Duchenne muscular dystrophy (DMD): Symptoms & care
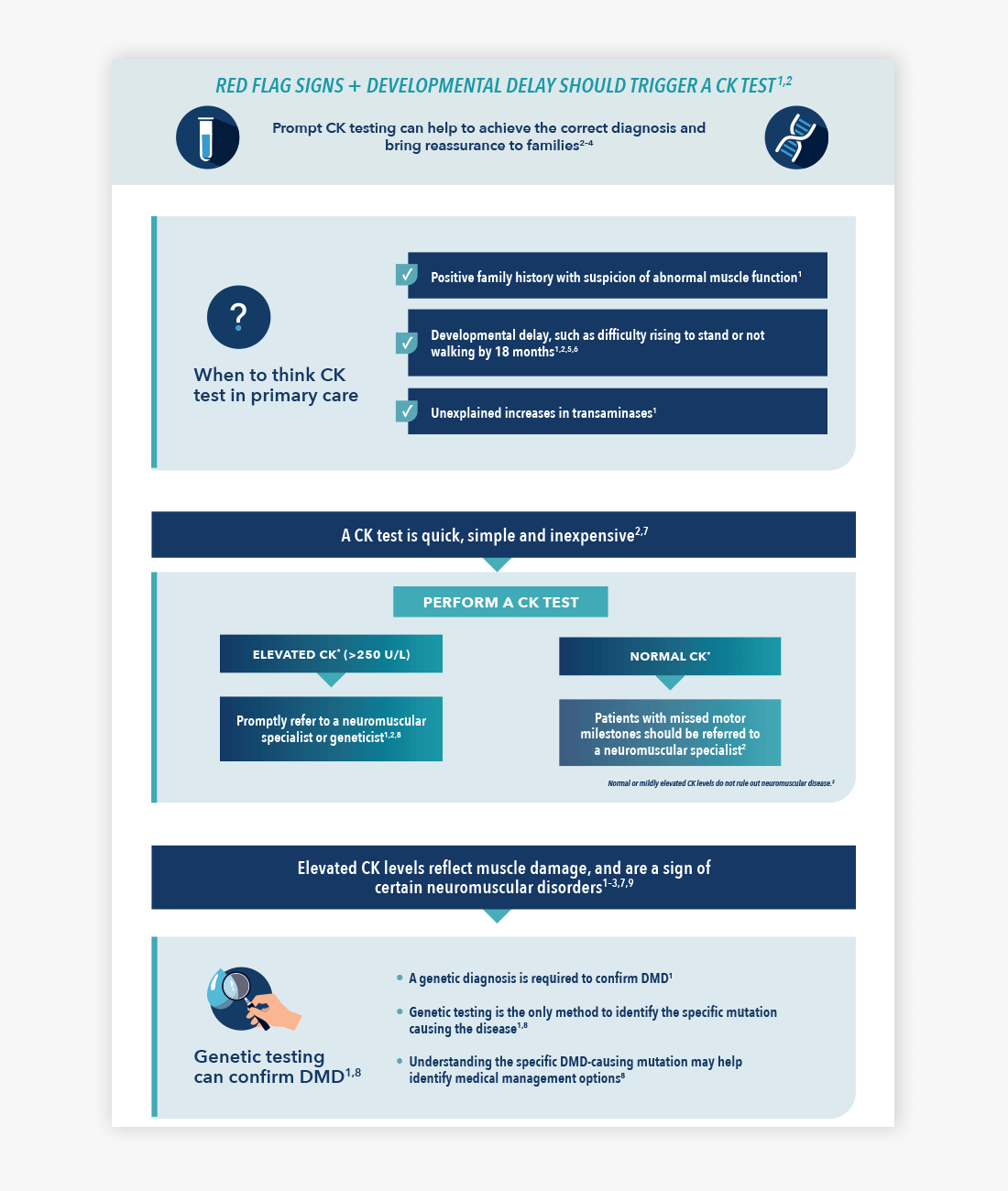
Download the infographic: When to test for DMD
Is it DMD? Download this handy infographic to find out about when to test for DMD


Duchenne muscular dystrophy symptoms
Early childhood signs and symptoms of DMD include weakness, clumsiness, Gowers’ sign, difficulties with stair-climbing or toe walking2
Duchenne muscular dystrophy diagnosis
An early and accurate diagnosis of DMD is a crucial aspect of care2


Genetic counselling
Genetic counselling offers many benefits for families diagnosed and living with DMD22—24


Duchenne muscular dystrophy treatment
The best outcomes for DMD patients are likely to result from the early application of DMD treatments25

The content on this page has been reviewed by Dr Christian Werner, Executive Director, Global Medical Affairs
Global Duchenne Muscular Dystrophy Lead, PTC Therapeutics.
This page has been through comprehensive review for informational and educational purposes. Find out more about our content review process.
References
- Goemans N, et al. Euro Neuro Rev. 2014;9(1):78–82.
- Birnkrant DJ, et al. Lancet Neurol. 2018;17:251–267.
- Marieb EN, Mitchell SJ. Human anatomy & physiology. 9th edition. Pearson; 2013.
- MDA. DMD muscular dystrophy (DMD): causes/inheritance. Available at: https://www.mda.org/disease/duchenne-muscular-dystrophy/causes-inheritance [Accessed November 2022].
- Nowak KJ, Davies KE. EMBO Rep. 2004;5(9):872–876.
- Pichavant C, et al. Mol Ther. 2011;19(5):830–840.
- Ervasti JM. Biochim Biophys Acta. 2007;1772(2):108–117.
- van Ruiten HJ, et al. Arch Dis Child. 2014;99:1074–1077.
- Lurio JG, et al. Am Fam Physician. 2015;91:38–44.
- Laing NG, et al. Clin Biochem Rev. 2011;32:129–134.
- Grounds MD, et al. Dis Model Mech. 2020;13(2):dmm043638.
- Blake DJ, et al. Physiol Rev. 2002;82:291–329.
- Sweeney HL. Developing skeletal muscle MRI/MRS as a biomarker for DMD therapeutic development. 2014 Annual Connect Conference, Chicago, IL, USA.
- Mendell JR, Lloyd-Puryear M. Muscle Nerve. 2013;48:21–26.
- Griffiths RD, Edwards RHT. Arch Dis Child. 1988;63:1256–1258.
- Bladen CL, et al. Hum Mutat. 2015;36:395–402
- Esterhuizen AI, et al. S Afr Med J. 2014;104:779–784.
- Kalman L, et al. J Mol Diagn. 2011;13:167–174.
- Ciafaloni E, et al. J Pediatr. 2009;155:380–385.
- Dent KM, et al. Am J Med Genet. 2005;134:295–298.
- Garcia S, et al. Int J Med Sci. 2014;11:988–993.
- Helderman-van den Enden AT, et al. Clin Genet. 2011;79:236–242.
- Institute of Medicine (US) Committee on Assessing Genetic Risks; Andrews LB, et al. editors. Assessing Genetic Risks: Implications for Health and Social Policy. Washington (DC): National Academies Press (US); 1994. 4, Issues in Genetic Counseling. Available at: https://www.ncbi.nlm.nih.gov/books/NBK236049/ [Accessed November 2022].
- Duchenne Connect. Genetic Counseling. Available at: https://www.duchenneconnect.org/genetic-counseling.html [Accessed November 2022].
- Aartsma-Rus A, et al. J Pediatr. 2019; 204:305–313.e4
© 2022 PTC Therapeutics.
GL-DMD-0709 | October 2023